Size and weight
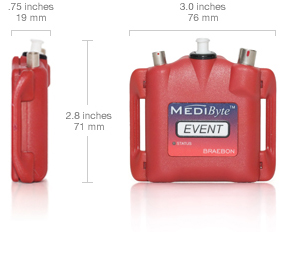
Connectors and controls

Battery and capacity

Available Channels
15 Total, 24 Bit ADC
Sampling rates exceed new guidelines
- Oximetry
- Heart Rate
- PPG (Photoplethysmograph)
- Pressure Flow
- Snore (derived from pressure)
- Thermal Flow
- Flow Limitation
- RIP Chest Effort
- RIP Abdomen Effort
- SUM
- Event Marker
- Body Position (internal sensor)
- EEG (250Hz), EOG (250Hz), EMG (250Hz), EKG (250Hz)
- EKG (250Hz), EMG (250Hz) or
Snoring Audio (2000Hz)
- Sound Level
- CPAP Flow
- CPAP Pressure
Snoring Audio (2000Hz)
Status indicator
- ●● Waiting to Collect
- ●● Collecting
- ●● Low Battery
- ●● No SpO2/Pulse Signal
Clearances & Certificates
- U.S. FDA
- Health Canada
- European CE
- IEC 60601
- Australian TGA
- ISO 13485
In the kit
- Oximetry finger probe
- Snore microphone Learn more
- 2 RIP respiratory effort belts Learn more
- 7 Oronasal PureFlow® cannulae Learn more
- EKG/EMG lead yoke adapter
- Preparation accessories
- Electrode Box
- Airflow thermistor Learn more
- Unparalelled BRAEBON support Learn more

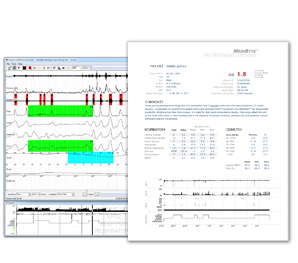
Analysis Software
- As simple as a few mouse clicks
- Meets guidelines for full disclosure
and simplified tools for manually
scoring or editing automated results
- Guided device configuration
with user-defined options
- Set the recorder to automatically
start at a specified date and time
- Computer-assisted scoring marks
all desaturations, respiratory,
PLM, and snoring events
- Powerful event editing
- Print any part of the recording
with a user-selected epoch size
- Export recordings in EDF for
research applications
- Microsoft Word® templates specific
to dental and cardiology sleep
medicine
and simplified tools for manually
scoring or editing automated results
with user-defined options
start at a specified date and time
all desaturations, respiratory,
PLM, and snoring events
with a user-selected epoch size
research applications
to dental and cardiology sleep
medicine
System Requirements
- PC with a USB 2.0 or 3.0 port
- 5 GB of hard disk space
- Windows 7, Windows 8.1 or Windows 10
*Product features and specifications are subject to change without notice and may not be exactly as shown.


